Functional Subnetworks. Structural Insight. Real Discovery.
Biological networks (and other natural networks) are shaped by many overlapping factors—obvious ones like genomics, cell type, disease state, and environment, and more nuanced ones, yet to be discovered. This makes it difficult to isolate what truly drives biological functions.
CNM simplifies the structure of these networks by identifying modules that work together across different contexts. It doesn’t just group modules based on similarity—it isolates and highlights the specific combinations that co-activate functions, consistently.
These combinations, called functional subnetworks, allow researchers to pinpoint the biological roles that emerge only when these multiple components interact. By analyzing which subnetworks appear across brain regions or disease states, CNM helps uncover which functions may be central to disease progression—and which may represent high-value targets for therapy.
Reveal the Hidden Structure Behind Complex Networks
Most tools analyze network connections—but few can explain why those connections exist.
CNM (Comprehension Normalization Method) isolates the underlying causes that shape a network’s structure. Instead of showing all variables at once, CNM separates their effects—so you can study one hidden driver at a time.
This gives researchers a clearer view of biological, social, or systemic networks—not just what’s connected, but why.
Comprehension Normalization Method for Networks
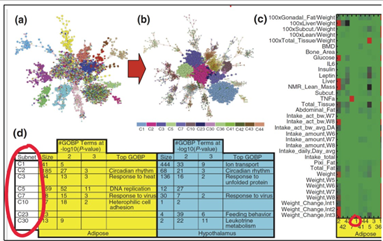
Tissue-to-Tissue Coexpression in Obesity (Mount Sinai Hospital)
We applied CNM to validate findings from a landmark study on inter-tissue gene regulation in obesity. That study identified coordinated gene modules spanning the hypothalamus, liver, and adipose tissue—revealing functions like circadian rhythm and immune regulation not visible in single-tissue analyses. CNM replicated the key results in minutes (rather than months), highlighting its efficiency and power in untangling complex cross-tissue networks with minimal data input.
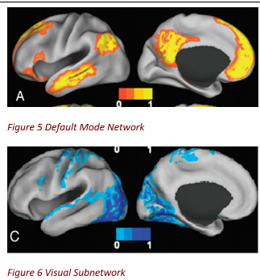
CNM successfully recovered two well-established brain subnetworks—the visual system and the default mode network—using only fMRI-derived network modules, without any prior knowledge of brain anatomy or disease.
This confirmed CNM’s blind ability to isolate functional brain architecture across starkly different mental states (rest vs. task) and populations (schizophrenic vs. healthy). While the findings were intentionally broad due to study design, the validation shows that CNM preserves known neurobiological structure—with the potential of uncovering nuanced, novel insights with more conditions and more closely targeted comparison datasets.
In Alzheimer’s research, CNM demonstrated its power to scale, analyzing coexpression networks from 19 brain regions in 342 pairwise comparisons.
This uncovered how different brain areas share functional architecture or diverge in disease. Leading Alzheimer’s researchers from Eli Lilly reviewed the results and were impressed by CNM’s ability to reveal coordinated biological patterns across regions. This work laid the foundation for our ongoing project identifying causal functions across Alzheimer’s brain networks.
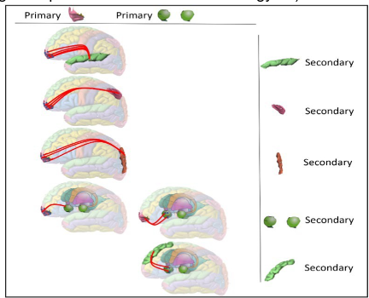
Current Project: Discovering Causal Functions Across Brain Regions in Alzheimer’s


We began by running CNM on a multi-region post-mortem Alzheimer’s dataset to uncover subnetworks—groups of modules that consistently co-regulate together within each brain region. For each subnetwork, we identified the biological functions enriched by its individual modules. But we went a step further: we kept only functions that were enriched by most or all of the modules within a subnetwork—functions that appear to require multiple components working together. These subnetwork-wide functions are visualized as color-coded groupings in the figure to the left.
Because we conducted this analysis across 17 brain regions, we next asked: are any of these functional subnetworks repeated across regions? In many cases, the same subnetwork function appeared in multiple areas. The red shading in the figure shows how often each function was enriched across regions. We consider these recurrent, multi-region functions especially promising—they may reflect shared drivers of Alzheimer’s progression.
We are now validating these subnetworks across independent datasets to test their robustness. If they hold up, the subnetwork-wide functions that appear across brain regions may represent credible targets for drug development. Most therapies aim to either activate or inhibit disease-driving functions. Identifying which **functions—not just genes—**are active in Alzheimer’s gives pharmaceutical companies a powerful starting point for therapeutic discovery.